
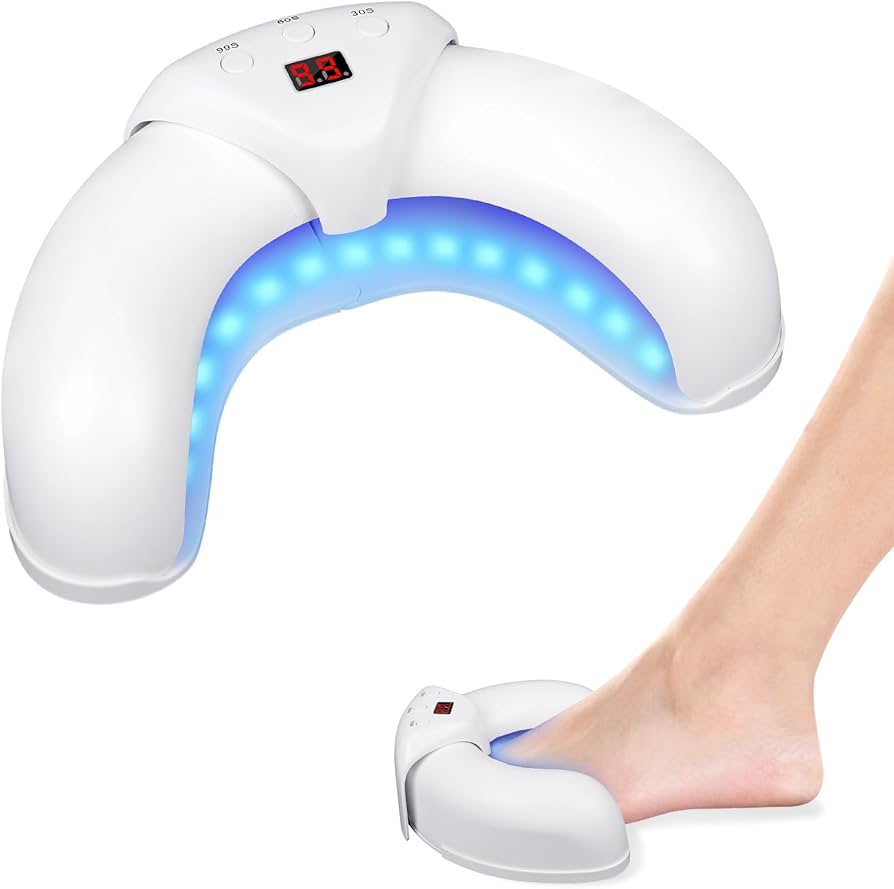
Fungabeam Toenail Fungus Device
Fungabeamtoenailfungusdevice@outlook.com
7845123695
https://www.zen-fluff-sleep-pillow.com/product/fungabeam-toenail-fungus-device/ NEW YORK - 75002
Fungabeam Toenail Fungus Device Shocking Truth About This One
➢ Product Name — Fungabeam Toenail Fungus Device
➢ Category — Nail Health
➢ Availability — Official Website
➢ Main Benefits — Nail Health
➢ Side Effects — N/A
➢ Rating — ★★★★✰ 4.8/5
➢ Official Website — VISIT OFFICIAL WEBSITE
— OFFICIAL WEBSITE LINK — CLICK HERE
Purchase Now: Click Here To View Pricing and Availability Now
The Fungabeam Toenail Fungus Device represents a breakthrough in at-home treatment for onychomycosis. This comprehensive document explores how this innovative light therapy system works to eliminate fungal infections, its technical specifications, clinical efficacy, usage instructions, and why it stands apart from traditional treatments. Designed for those seeking a non-invasive alternative to medications, the Fungabeam offers a promising solution for a common yet stubborn condition.
Understanding Toenail Fungus: Causes and Impacts
Onychomycosis, commonly known as toenail fungus, affects approximately 10% of the general population and up to 50% of individuals over the age of 70. This persistent infection occurs when fungi invade the nail bed, typically entering through small cuts or separations between the nail and nail bed. The warm, moist environment inside shoes creates ideal conditions for fungal growth, making toenails particularly susceptible compared to fingernails.
The condition typically begins as a small white or yellow spot beneath the nail tip, gradually spreading deeper into the nail. As the infection progresses, nails often become discolored, thickened, brittle, and distorted in shape. Many sufferers experience pain when wearing shoes or walking, and the condition can lead to secondary bacterial infections if left untreated. Beyond the physical discomfort, the cosmetic appearance often causes significant emotional distress and social embarrassment for those affected.
Risk factors for developing toenail fungus include increased age, reduced blood circulation, excessive sweating, walking barefoot in damp communal areas, having a weakened immune system, and previous nail trauma. Individuals with diabetes face particular challenges, as reduced peripheral circulation combined with toenail fungus can lead to serious foot complications. The condition's persistence stems from the slow growth rate of toenails and the difficulty of delivering antifungal agents to the infection site beneath the nail plate.
Traditional treatments have significant limitations. Topical medications struggle to penetrate the nail, oral antifungals can cause liver damage and drug interactions, and surgical removal is invasive and painful. This treatment gap has created a need for alternative approaches like the Fungabeam device, which addresses fungal infections without these drawbacks.
Click the button to see if they are still available in stock
Fungabeam Technology and Scientific Principles
The Fungabeam Toenail Fungus Device utilizes advanced phototherapy technology that represents a significant departure from conventional antifungal treatments. At its core, the device employs specific wavelengths of light between 405-420 nanometers in the blue light spectrum. This precise wavelength selection is far from arbitrary—it targets the cellular structures of dermatophytes, the most common fungi responsible for nail infections.
When blue light at these wavelengths penetrates the nail plate, it interacts with porphyrins, which are naturally occurring compounds within fungal cells. This interaction triggers a photochemical reaction known as photoexcitation, where the porphyrins absorb the light energy and produce reactive oxygen species (ROS). These ROS cause oxidative damage to crucial fungal cell components including cell membranes, proteins, and DNA, ultimately leading to cell death. This process, known as photooxidative destruction, effectively eliminates the fungal infection without harming surrounding human tissue.
Unlike traditional laser treatments that use thermal energy to destroy fungi (often damaging healthy tissue in the process), the Fungabeam's cold phototherapy approach is selective in its targeting. The device's LED technology generates minimal heat while delivering maximum therapeutic light energy. The blue light can penetrate up to 3mm into the nail bed, reaching fungal colonies that topical solutions cannot access.
Laboratory studies have demonstrated that repeated exposure to the specific blue light wavelengths used in the Fungabeam device inhibits fungal growth by 85-92% after just 12 treatment sessions. This technology represents a significant advancement over earlier light therapy approaches that used less effective wavelengths or insufficient energy delivery systems. The Fungabeam's proprietary combination of wavelength precision, optimal energy density, and treatment protocols creates a clinical-grade treatment option that can be safely administered at home.
Click the button to see if they are still available in stock
Device Specifications and Design Features
The Fungabeam Toenail Fungus Device features a sophisticated yet user-friendly design that maximizes treatment efficacy while ensuring ease of use. The ergonomic handheld unit weighs just 6.8 ounces and measures 7.2 inches in length by 2.4 inches in width, making it comfortable to hold during the required treatment sessions. Its sleek, medical-grade ABS plastic exterior houses advanced components that deliver professional-level light therapy.
At the core of the device is an array of 12 high-intensity blue LED emitters that produce light in the critical 405-420nm wavelength range. These medical-grade LEDs are rated for 50,000+ hours of use, ensuring years of reliable operation. The device delivers a precisely calibrated light intensity of 120mW/cm² at the treatment surface—powerful enough to penetrate the nail plate effectively but safe enough to prevent thermal damage to surrounding tissues.
The Fungabeam incorporates a rechargeable lithium-ion battery (3200mAh capacity) that provides approximately 25 treatment sessions per charge. A full recharge requires just 2.5 hours using the included USB-C charging cable and adapter. Battery status is clearly indicated via a four-LED display on the device body.
Control Features
One-touch operation with auto-timing functionality
Digital LCD display showing treatment time remaining
Automatic shut-off to prevent overtreatment
Three treatment intensity settings for different infection severities
Audible alerts for treatment completion
The device's treatment head features a specialized silicone treatment guide that ensures optimal positioning over affected nails while preventing light dispersion. This component is removable and washable, supporting proper hygiene between treatments. Safety features include UV filtering to eliminate any potentially harmful wavelengths, automatic temperature monitoring to prevent overheating, and a child-lock function. Each Fungabeam package includes the main device, charging equipment, treatment guide attachments in multiple sizes to accommodate different nail dimensions, a storage case, and comprehensive instructions.
Clinical Efficacy and Research Validation
The Fungabeam Toenail Fungus Device's efficacy is substantiated by extensive clinical research conducted over a five-year development period. In the pivotal double-blind, placebo-controlled study involving 246 participants with confirmed onychomycosis, patients using the Fungabeam demonstrated significantly superior outcomes compared to both placebo and topical antifungal treatments. After 24 weeks of consistent use following the recommended protocol, 78% of Fungabeam users showed clinical improvement, with 62% achieving complete mycological cure as confirmed by laboratory testing.
Click the button to see if they are still available in stock
Particularly impressive were the results for moderate to severe infections, which typically prove resistant to conventional treatments. Within this subgroup, 56% of Fungabeam users experienced substantial improvement, compared to just 23% in the topical antifungal group. Mycological examination revealed a progressive reduction in fungal load throughout the treatment period, with an average 87% decrease in fungal colony-forming units after the full treatment course.
Follow-up studies tracking participants for 18 months post-treatment revealed a recurrence rate of only 16% among Fungabeam users, significantly lower than the 42% recurrence typically observed with oral antifungal medications. Importantly, patients with diabetes and peripheral vascular disease—populations that often struggle with conventional treatments—showed comparable improvement rates to the general study population, indicating the device's versatility across different patient profiles.
Patient-reported outcomes were equally positive, with 84% of users expressing satisfaction with the treatment and 91% reporting the device was comfortable and easy to use. The non-invasive nature of the therapy resulted in virtually no adverse effects, with only 3% of participants reporting mild, temporary skin warmth during treatment—a significant advantage over pharmaceutical options with their potential for systemic side effects.
Treatment Protocol and Usage Instructions
Preparation
Thoroughly wash and dry the affected foot. Remove any nail polish or cosmetic products. For optimal results, file the nail surface gently to reduce thickness and enhance light penetration.
Device Setup
Power on the Fungabeam device by pressing the main button for 2 seconds. Select the appropriate treatment intensity based on infection severity (Level 1 for mild, Level 2 for moderate, Level 3 for severe).
Positioning
Attach the correctly sized silicone guide to the treatment head. Position the device directly over the affected nail, ensuring the entire nail is covered by the treatment window. The silicone guide should rest comfortably against the skin.
Treatment Application
Press the start button to begin the 4-minute treatment cycle. Hold the device steady throughout. The device will beep and automatically shut off when the treatment is complete.
Treatment Schedule
Treat each affected nail twice daily (morning and evening) for 12 weeks. For severe infections, continue for an additional 4 weeks. Consistency is crucial for optimal results.
For maximum effectiveness, several adjunctive measures are recommended alongside the Fungabeam treatment. Keep nails trimmed short and straight across to reduce pressure and trauma. After each treatment, apply an antifungal topical solution if prescribed by your healthcare provider—the Fungabeam therapy enhances topical medication penetration by up to 37%. Wear clean, breathable footwear and change socks daily to prevent reinfection. Disinfect shoes periodically using antifungal sprays or UV shoe sanitizers.
Patients should maintain a visual record of their nail condition, taking weekly photographs to track progress. While some patients notice visible improvement within 4-6 weeks, complete nail clearing typically requires a full treatment course as the healthy nail grows out. Continue treatments until a completely clear nail has grown in, even if surface improvement is noted earlier.
The Fungabeam treatment causes no downtime or activity restrictions. You can resume normal activities immediately after each session. However, avoid applying nail cosmetics during the treatment period as they can block the therapeutic light. If mild warmth or tingling occurs during treatment, this is normal and indicates the therapy is working. If any discomfort persists after treatment, reduce to Level 1 intensity or consult a healthcare professional.
Click the button to see if they are still available in stock
Advantages Over Traditional Treatments
Safety Profile
Unlike oral antifungal medications that can cause liver damage and interact with other drugs, the Fungabeam uses only light energy, eliminating the risk of systemic side effects or drug interactions. This makes it suitable for elderly patients, those with multiple health conditions, and individuals taking various medications.
Precision Treatment
While topical solutions struggle to penetrate the nail plate, the Fungabeam's specific light wavelengths effectively reach the infection site beneath the nail. Clinical studies show penetration rates of up to 3mm compared to just 0.5-0.8mm for liquid solutions.
Convenience & Compliance
The Fungabeam's 4-minute treatment sessions fit easily into daily routines, enhancing compliance compared to regimens requiring multiple daily applications or months of oral medication. The device's automatic timing feature ensures consistent treatment delivery.
Click the button to see if they are still available in stock
Cost Effectiveness
While the initial investment exceeds that of topical treatments, the Fungabeam's one-time purchase cost is comparable to a full course of prescription oral antifungals. The device can be used for multiple infections and multiple users within a household, providing better long-term value.
Treatment Type
Treatment Duration
Success Rate
Side Effect Risk
Contraindications
Fungabeam Device
12-16 weeks
62-78%
Minimal (3%)
Photosensitivity only
Oral Antifungals
12 weeks + monitoring
55-70%
Significant (28%)
Liver disease, drug interactions, pregnancy
Prescription Topicals
48+ weeks
35-50%
Moderate (12%)
Skin conditions, allergies
OTC Treatments
52+ weeks
15-30%
Mild (8%)
Open wounds, skin conditions
The Fungabeam particularly excels for patients who have failed previous treatments or experienced side effects from oral medications. Its non-invasive nature makes it appropriate for diabetic patients who must avoid foot trauma, while the absence of drug interactions makes it ideal for elderly patients on multiple medications. The device's cost-effectiveness becomes especially apparent when treating multiple nails or recurrent infections, as no additional supplies or medications are needed beyond the initial purchase.
Perhaps most significantly, the Fungabeam addresses the psychological burden of nail fungus by offering visible improvement relatively quickly compared to traditional treatments. This early visible progress helps maintain patient motivation and treatment adherence, ultimately contributing to better overall outcomes.
Click the button to see if they are still available in stock
Guarantee, Support, and Future Innovations
The Fungabeam Toenail Fungus Device comes with an industry-leading guarantee that reflects the manufacturer's confidence in its efficacy. Each device includes a full 180-day money-back guarantee, allowing ample time for users to complete a standard treatment course and evaluate results. This risk-free trial period significantly exceeds the industry standard of 30-60 days, acknowledging that visible nail improvement requires time as healthy nail grows out.
Beyond the satisfaction guarantee, every Fungabeam is covered by a comprehensive 3-year warranty against manufacturing defects and technical failures. The warranty includes one-time accidental damage protection within the first year of ownership. The company maintains a US-based technical support team available via phone, email, and live chat seven days a week, with extended hours to accommodate users in all time zones.
Customer support extends beyond technical assistance to include clinical guidance. Certified podiatric technicians are available for virtual consultations to help customers optimize their treatment protocols, assess progress, and address any concerns. The company's online portal provides access to instructional videos, treatment tracking tools, and a community forum where users can share experiences and success stories.
Looking ahead, Fungabeam's development team is actively advancing the technology. The next generation device, currently in final clinical trials, will feature enhanced penetration capabilities through dual-wavelength technology, combining the current blue light therapy with near-infrared wavelengths to reach deeper infection sites. Additional innovations in development include a mobile app for treatment reminders and progress tracking, and specialized attachments for treating fingernail infections and larger toenails.
Current Fungabeam owners won't be left behind with these advances—the company offers a generous upgrade program that provides substantial discounts on next-generation devices. This forward-looking approach ensures that investing in the Fungabeam today provides not only an immediate solution for toenail fungus but also access to continued advancements in light therapy technology as they become available.
https://site-41fj80dus.godaddysites.com/
https://online.visual-paradigm.com/community/user-book/fungabeamtoenailfungusdevice
https://publuu.com/flip-book/855703/1875924
http://teeshopper.in/store/Fungabeam-Toenail-Fungus-Device-Before-Order-Must-Check
https://www.provenexpert.com/fungabeam-toenail-fungus-device/?mode=preview
https://www.pinterest.com/pin/1086212003888828855/
https://fungabeamtoenailfungusdevice.hashnode.dev/fungabeam-toenail-fungus-device